Medical Devices - A Computer Science Presentation
Presented and Made By:
Matias Suxo
ICS3U - Mr.kzebarth
Policies
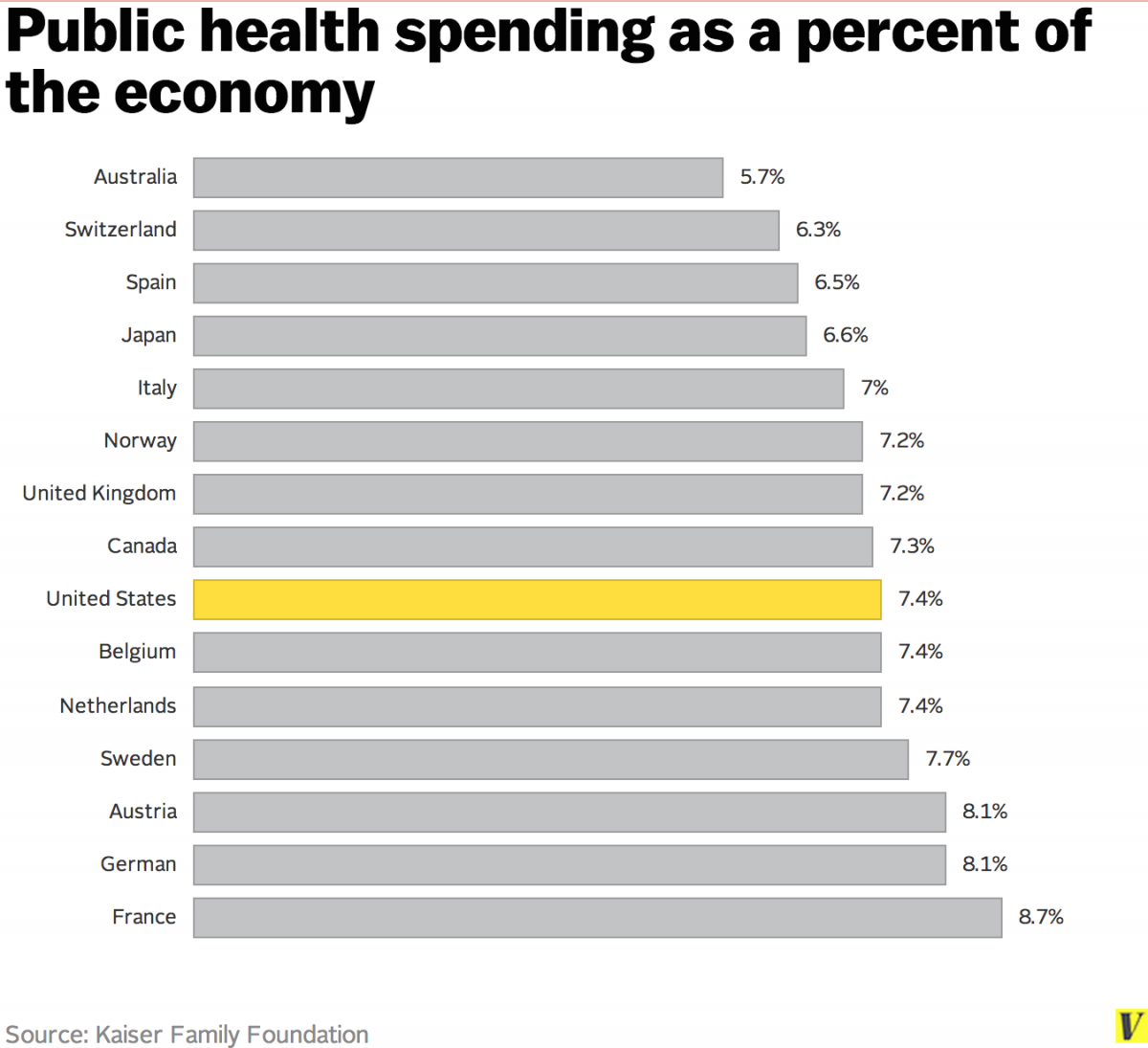
Policies relating Medical Devices
With the possible outcomes some Medical Devices can give the Canadian and majority governements have heavy regulations and testing when using and selling Medical Devices. Before a risky Device is made it needs to get various approvals that may never happen or take a long time even when the device itself is not as harmful as thought. Elon Musk's company Neuralink (A microchip implant developpment company) is a good example about this, the device at its first stage is rather harmless and is only meant to restore brain malfunction and defects such as alzheimer's or bad eyesight but in order to get FDA (U.S based, in Canada it is CDA) approval it will require many years due to its new technology. To summarise, many regulations are enforced to maintain consumer safety from products to hospitals where doctors MUST tell you ALL risks involved in procedures and devices. Graphic on the left shows a public health spending


